44 medication labels must include
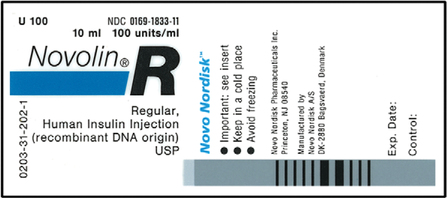
Reading Medication Labels | Basicmedical Key Reading Medication Labels Objectives After reviewing this chapter, you should be able to identify: 1. The trade and generic names of medications 2. The dosage strength of medications 3. The form in which a medication is supplied 4. The total volume of a medication container where indicated 5. Drug labeling, Information about Drug labeling - FAQs Each product must contain a label with "Supplement Facts" in bold letters onthe front panel. This is the manufacturer's opportunity to identify the product. Below "Supplement Facts," the panel must state the serving size. This isdetermined by the manufacturer with no input from the FDA.
How to Read Over-the-Counter and Prescription Drug Labels - Drugwatch.com Some labels include a seventh section with a phone number to call if you have questions or comments. The Drug Facts label for the over-the-counter drug acetaminophen, known by the brand name Tylenol, includes information about ingredients, uses, warnings and directions. Active Ingredient and Purpose.
Medication labels must include
How to Label Prescription Medication for Veterinary Patients A label should include the following components: The name of the veterinary practice, its address, and contact information The veterinarian's name, the patient's name and species, and the client's... Safe Labeling Helps Prevent OR Medication Errors - OR Today Label information must include a medication's name and strength as well as amount when medications are mixed (as with antibiotic irrigations, tumescent and heparin solutions, and epinephrine). The unit of measure — percent, grams, milliliters, or units — must be recorded along with the date the medication is prepared. A Guide To Veterinary Prescription Label Requirements What Is Required On A Veterinary Prescription Label As shown in the above example, the actual container must include the following information: The name of the veterinary practice, its address, and contact information The veterinarian's name, the patient's name and species, and the client's last name
Medication labels must include. Guidance Document: Labelling of Pharmaceutical Drugs for Human Use (1.4) When the composition of the drug varies from one lot to another, the outer label must include a reference to all non-medicinal ingredient alternatives that may be present in the drug, ... Claims on drug product labels that include market share, sale, consumer and patient use/ choice, or preference must be supported by adequate studies ... Pharmaceutical Labeling 101: FDA Regulations Guide These include drugs like analgesics, anti-inflammatory agents, antibacterial, anticonvulsants, and others. The substance is used in the diagnosis, mitigation, cure, treatment, or prevention of diseases. This category also includes supplements. The substance is a component of medication but not a part of a medical device. How to read prescription drug labels - BeMedwise Whenever you are prescribed a medication, you should read and follow the information in the medication's "label" in order to ensure your safety. All prescription medicine containers include information on the label including the patient's name, the name of the medicine, dosage and instructions on how often to take the medicine. What's on a prescription label? - Knowledge is the best medicine It uniquely identifies all drug products sold in a dosage form in Canada and is located on the label of prescription and over-the-counter drug products that have been evaluated and authorized for sale in Canada. A DIN uniquely identifies the following product characteristics: manufacturer; product name; active ingredient (s); strength (s) of ...
Chapter 5: Prescriptions and Labels Flashcards | Quizlet Drug Labels Regulated by the Food and Drug Administration (FDA), which determines what needs to be on the label Dispensing pharmacist's label must include: Pharmacy name, address, and phone number Dispensing date Dispensing date may differ from the date on the prescription. Rx number, which identifies this unique prescription in the computer system What's on my medicine label? - Therapeutic Goods Administration (TGA) A doctor or pharmacist may include warnings for their patient on a prescription medicine by applying an additional label. Declarations. Some substances/ingredients found in medicines must be declared on the label. For example, potential allergens such as egg and fish must be declared if they are likely to be in the medicine. Medicines: packaging, labelling and patient information leaflets Labels must include warnings for safe use of the medicine. All products that contain paracetamol must include statutory warnings. Additional warning statements must be included on the packaging of... OTC Labeling Requirements - FindLaw To qualify for the modifications, a package must be considered "small"-that is, more than 60 percent of the total surface area available for labeling is needed to accommodate the FDA required labeling. The FDA interprets available surface area to include the entire container, except for flanges on the tops and bottoms of cans and the shoulders ...
The Over-the-Counter Medicine Label: Take a Look | FDA All nonprescription, over-the-counter (OTC) medicine labels have detailed usage and warning information so consumers can properly choose and use the products. Below is an example of what the OTC... Pharmaceutical Labeling: Requirements & Guidelines - CTM Labeling Systems To meet today's FDA regulations, labeling information on drugs must include the following in this order: - Product Name - Drug Facts Table - Active Ingredients - Purpose and Use - Warnings - Directions - Allergic Reactions - Inactive Ingredients Labeling Information | Drug Products | FDA For more information on labeling, including Physician Labeling Rule (PLR) requirements, guidances, presentations, sample templates and format tools, and established pharmacologic class (EPC ... NCBOP - Pharmacist FAQs 10. "Filled by" or "dispensed by" with the name of the dispensing pharmacist. The name must include, at a minimum, the first initial and full last name of the dispensing pharmacist. 11. If the dispensed drug is a "tranquilizer or sedative," it should bear the warning "The consumption of alcoholic beverages while on this medication ...
Ch 8 Pharmacy Flashcards | Quizlet A legal prescription label must include all of the following except: A. Directions for use B. Date the prescription was dispensed C. Name, address, and telephone number of the prescriber D. Name, address, and telephone number of the dispensing pharmacy Name, address, and telephone number of the prescriber
FDA Says Drug Labels Must Include Clear Guidance for ... - Healthline Starting June 30, new drug labels will have categories for "Pregnancy," "Lactation," and "Females and Males of Reproductive Potential." "Pregnancy" will include information on ...
A Guide To Veterinary Prescription Label Requirements What Is Required On A Veterinary Prescription Label As shown in the above example, the actual container must include the following information: The name of the veterinary practice, its address, and contact information The veterinarian's name, the patient's name and species, and the client's last name
Safe Labeling Helps Prevent OR Medication Errors - OR Today Label information must include a medication's name and strength as well as amount when medications are mixed (as with antibiotic irrigations, tumescent and heparin solutions, and epinephrine). The unit of measure — percent, grams, milliliters, or units — must be recorded along with the date the medication is prepared.
How to Label Prescription Medication for Veterinary Patients A label should include the following components: The name of the veterinary practice, its address, and contact information The veterinarian's name, the patient's name and species, and the client's...











Post a Comment for "44 medication labels must include"